Quality Assurance
Quality is our Commitment
GLP Practices
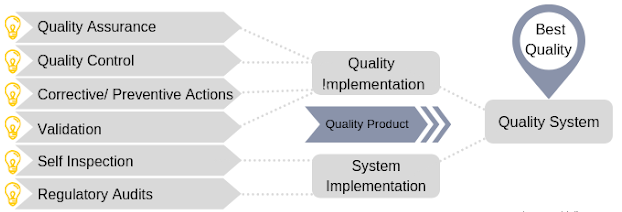
Quality Assurance at Agror means totality of arrangement for quality drugs which includes cGMP Compliance Monitoring at Production, Quality Control, Utilities, Material Management, Administration & Regulatory Compliance which has direct & indirect impact on product Quality, Safety, Purity & Efficacy.
Quality Assurance at Agror is independent & committed to ensure that the entire operations of its manufacturing facility meets the required cGMP standards and products manufactured therein conforms to the internationally accepted standards throughout its shelf life. Execution of Validation Master Plan through Annual Validation Plan and we at Agror are successively complying the validation requirement like:
- Utilities/System Qualification (HVAC, Water, Compressed Air, Pure Steam)
- Equipment Qualification
- Analytical Method Validation
- Process Validation
- Aseptic Process Validation
- Cleaning Validation
- Computer System Validation
- Supplier Qualification
- Internal Audit/Self Inspection & cGMP Trainings
- Batch Release Authorization through review of documentation by qualified personnel.
Supplier Qualification with coordination of Supply Chain Management is base of quality raw materials & packaging components. QA has emphasis upon Analysis of Data through Annual Product Review (APR), Quality Risk Management (QRM), Handling & Investigation of Compliant, Deviation & Change Control, Self Inspection/Technical Audit & cGMP Training Plan to Worker & Executive Staff. QA has microscopic role at Agror & everything has to pass through it.
About Agror
Agror Pharma is the state-of-the-art Cephalosporin manufacturing facility which started its operations in Islamabad, Pakistan having operational designs meeting US FDA, EMA & WHO cGMP standards.
Helpful Links
- About Agror
- Production
- Quality Control
- Contact Us